Kosmetischer Rohstoff mit aufhellender Wirkung - Arbutin

Was ist Arbutin
Arbutin, auch bekannt als Arbutin, hat die chemische Bezeichnung 4-Hydroxyphenyl-D-glucopyranosid und eine relative Molekularmasse von 272,25. Es ist ein natürlich vorkommendes Glykosid, das aus grünen Pflanzen gewonnen wird und Bestandteil vieler resistenter gefriergetrockneter Pflanzen wie Weizen, Birnen und Bärentrauben aus der Familie der Rhododendrongewächse ist, die reich an gelösten Stoffen sind. Sie erscheinen als weiße, nadelförmige Kristalle oder Pulver. Sie sind leicht löslich in heißem Wasser, Methanol, Ethanol, Propylenglykol und Glycerin und unlöslich in Ether, Chloroform und Petroleum. Lösungsmittel wie Ether.
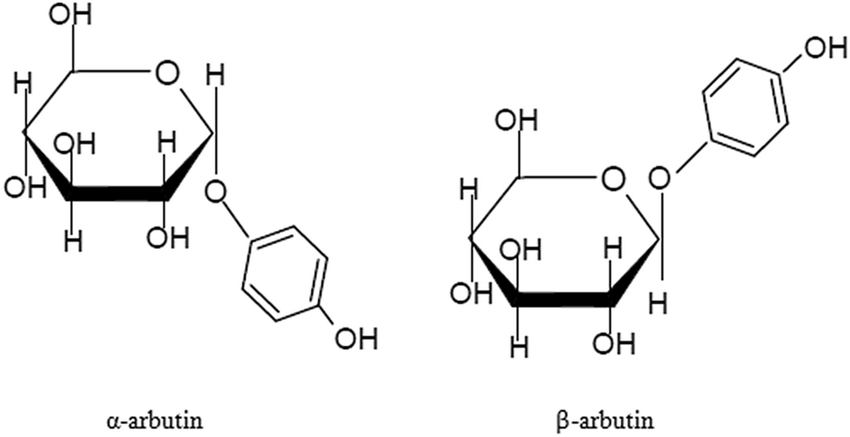
Arbutin lassen sich aufgrund unterschiedlicher Strukturen in den α-Typ und den β-Typ unterteilen. Der chemische Name von α-Arbutin ist 4-Hydroxyphenyl-α-D-glucopyranosid, und der chemische Name von β-Arbutin ist 4-Hydroxyphenyl-β-D-glucopyranosid. α-Arbutin ist das Epimer von β-Arbutin, und die Richtung seiner glykosidischen Bindung im Raum ist entgegengesetzt zu der von β-Arbutin (siehe Abbildung für Details)
Der Aufhellungsmechanismus von Arbutin
Der Gehalt und die Verteilung von Melanin sind die wichtigsten Faktoren, die die Tiefe der Hautfarbe bestimmen. Melanin wird in Melanozyten in der Basalschicht der Epidermis der Haut gebildet. Es wird schließlich aus Tyrosin unter der Einwirkung von Tyrosinase durch eine Reihe komplexer biochemischer Reaktionen gebildet. Es wird von der Basalschicht über Synapsen von innen nach außen auf die äußere Schicht der Epidermis übertragen. Färben Sie die Haut.
Tyrosinase hat eine Tyrosin-Hydroxylase-Aktivität (katalysiert Tyrosin zur Herstellung von Dopa) und eine Dopa-Oxidase-Aktivität (katalysiert Dopa zur Herstellung von Dopaquinon). Im Prozess der Melaninbildung ist sie das wichtigste geschwindigkeitsbeschränkende Enzym. Seine Aktivität bestimmt die Menge des gebildeten Melanins.
Arbutin ist ein Tyrosinase-Inhibitor, der die Tyrosinase-Aktivität wirksam hemmen kann, ohne die Konzentration der Zellproliferation zu beeinträchtigen. Es konkurriert um die Bindung von Dopa durch seine direkte Bindung an die Tyrosinase. Site, blockiert die Synthese von Dopa und Dopaquinon, wodurch mit Melanozyten stören und hemmt die Produktion von Melanin. Gleichzeitig hat es auch die Funktion, gebildetes Melanin zu verdünnen, den Abbau und die Ausscheidung von Melanin zu beschleunigen, die Pigmentierung der Haut zu verringern und Flecken und Sommersprossen zu entfernen.
Verfahren zur Herstellung von Arbutin
natürliche Pflanzenextraktionsmethode
Bei dieser Methode werden hauptsächlich Pflanzenblätter der Gattung Ursi als Rohstoffe verwendet, und zur Gewinnung des Arbutin-Extrakts werden organische Lösungsmittelextraktion, Extraktion, Säulenchromatographie und andere Trennungs- und Reinigungsverfahren eingesetzt. Bereits 1930 wurde berichtet, dass Arbutin in den Blättern des Felsenkrauts enthalten ist. Spätere Studien haben bestätigt, dass Arbutin auch in den Blättern des schwarzen Reisbaums, der Heidelbeere, der Bärentraube und des Birnbaums vorkommt. glykoside.
Da der Gehalt an Arbutin in Pflanzen sehr gering ist, der Extraktionsprozess relativ komplex ist und der Reinheitsgrad des Extrakts nicht hoch ist, hat die Pflanzenextraktionsmethode mit der Entwicklung anderer Zubereitungsmethoden allmählich ihren Wettbewerbsvorteil verloren.
Pflanzengewebekultur
Die Methode der Pflanzengewebekultur nutzt die Glykosylierungsfähigkeit der Pflanzenzellen, um Hydrochinon in Arbutin umzuwandeln. Im Vergleich zu Pflanzenextraktionsverfahren ist die Effizienz der Arbutingewinnung mit Hilfe von Pflanzengewebekulturverfahren wesentlich höher. Bei der Anwendung dieser Methode sind die Auswahl eines effizienten Pflanzengewebekulturmediums und die Festlegung geeigneter Kulturbedingungen entscheidend.
Die bei der Methode der Pflanzengewebekultur verwendeten Rohstoffe sind sauber, die Umwandlungsrate ist hoch und die Produktion ist umweltfreundlich. Allerdings ist der Produktionszyklus lang, die Trennung und Reinigung kompliziert, und die industrielle Entwicklung ist relativ unreif. Das weitere Verständnis des Wachstumsmechanismus von Pflanzenzellen, die Klärung der wichtigsten Einflussfaktoren des Syntheseprozesses, die Verkürzung des Produktionszyklus und die Verbesserung der Ausbeute sind die wichtigsten Fragen, die bei der Anwendung dieser Methode gelöst werden müssen.
Verfahren zur Enzymsynthese
Bei der Enzymsynthesemethode wird hauptsächlich Glykosyltransferase oder Glykosidase als Katalysator verwendet, um Glykosyltransfer und umgekehrte Hydrolysereaktionen zur Synthese von Glykosiden zu katalysieren, d. h. Arbutin wird aus Hydrochinon und Glukose unter der Katalyse von Glykosidase gewonnen.
Die Enzymsynthesemethode hat einen einfachen Prozess, eine hohe Syntheseeffizienz und sehr optimistische Entwicklungsaussichten. Durch die eingehende Erforschung dieser Methode in den letzten Jahren wurden immer mehr geeignete Zymogene entdeckt, und die Syntheserate von Arbutin wird ebenfalls immer höher. Es wird davon ausgegangen, dass diese Methode eine der wichtigsten Forschungsrichtungen für die Synthese von Arbutin in der Zukunft sein wird. eins.
chemische Synthese
Im Allgemeinen werden bei der chemischen Synthese von Arbutin Glukose und Hydrochinon als Ausgangsstoffe verwendet. Nachdem die beiden entsprechend geschützt sind, werden sie einer Glykosidierungsreaktion unterzogen und anschließend die Schutzgruppe entfernt. Die chemische Synthesemethode hat sich aufgrund der besseren Qualität des Syntheseprodukts und der niedrigeren Produktionskosten zur wichtigsten Methode für die Herstellung von Arbutin entwickelt und wird im In- und Ausland industriell produziert.
Gegenwärtig wird in China im Allgemeinen wasserfreie Glukose als Rohstoff verwendet, und β-Arbutin wird durch Acylierungsschutz, katalytische Kondensation und alkalische Hydrolyse hergestellt. Mit der kontinuierlichen Entwicklung der Synthesetechnologie wurden in den letzten Jahren die Schritte für die inländische Synthese von Arbutin schrittweise vereinfacht, die Syntheserate wurde kontinuierlich verbessert, und die Qualität hat das internationale Spitzenniveau erreicht. Aufgrund der geringen Stereoselektivität des Produkts in der chemischen Synthese ist jedoch noch weitere Forschung erforderlich, um eine effiziente und spezifische chemische Synthesemethode zur Herstellung von α-Arbutin zu finden.
() ()


